Between Supplement Hype and Evidence: A Clinical Guide for Informed Use

Demand for dietary supplements — including vitamins, minerals, and botanical products — continues to rise rapidly in clinical practice. Driven largely by aggressive marketing, social media influence, and growing public interest in “natural” health solutions, many patients now view supplements as essential tools for disease prevention and overall wellness. However, scientific evidence often fails to support the high expectations surrounding these products, particularly for individuals who already follow balanced diets.
For clinicians, this growing popularity presents both challenges and opportunities: challenges in correcting misinformation and preventing unnecessary or harmful use, and opportunities to guide patients toward evidence-based decisions.
The Expanding Supplement Market and Patient Expectations
Dietary supplements are widely perceived as safe, natural, and beneficial. Patients frequently assume that taking vitamins or herbal products can enhance immunity, improve energy, prevent chronic disease, or compensate for dietary shortcomings. As a result, many individuals self-prescribe supplements without professional guidance.
In reality, for most healthy people with adequate nutrition, routine supplementation offers little additional benefit. Large clinical trials have repeatedly shown that many commonly used vitamins and minerals do not significantly reduce the risk of cardiovascular disease, cancer, or overall mortality when taken by individuals without documented deficiencies.
Evidence Gaps and Inconsistent Product Quality
One of the major concerns surrounding supplements is the inconsistency of scientific evidence. While some nutrients are clearly beneficial in specific deficiency states, evidence supporting widespread use in the general population remains limited or inconclusive.
Product quality adds another layer of complexity. Unlike prescription medications, dietary supplements are not subject to rigorous pre-market approval in many countries. This can result in:
-
Variable ingredient concentrations
-
Contamination with heavy metals or undeclared pharmaceuticals
-
Mislabeled or misleading claims
Such inconsistencies make it difficult for clinicians and patients alike to determine which products are safe and effective.
When Supplementation Is Appropriate
Despite the limitations, supplements can play an important role in certain clinical scenarios. Evidence supports targeted supplementation in populations at risk for deficiency, including:
-
Vitamin D in individuals with limited sun exposure or bone health concerns
-
Iron in patients with confirmed iron-deficiency anemia
-
Vitamin B12 in older adults or those following strict plant-based diets
-
Folic acid during pregnancy to prevent neural tube defects
In these cases, supplementation should be guided by clinical evaluation, laboratory testing when appropriate, and individualized patient needs.
Identifying High-Quality Products
When supplementation is indicated, clinicians can help patients choose safer options by recommending products that:
-
Have third-party quality testing or certification
-
Provide clear ingredient lists and standardized dosages
-
Avoid exaggerated or disease-curing claims
-
Are produced by reputable manufacturers
Encouraging patients to disclose all supplement use is also critical, as some products may interact with prescription medications or exacerbate underlying conditions.
Avoiding Low-Value or Misleading Supplements
Many supplements marketed for “immune boosting,” “detoxification,” or rapid disease prevention lack credible scientific backing. In some cases, these products may create false reassurance, delay proper medical care, or expose patients to unnecessary risks.
Clinicians play a vital role in helping patients understand that “natural” does not automatically mean “safe” or “effective,” and that more is not always better when it comes to supplementation.
Bridging the Gap Between Marketing and Medicine
As supplement use becomes increasingly mainstream, evidence-based guidance is essential. Clinicians should aim to engage patients in open, nonjudgmental discussions, focusing on realistic expectations, scientific evidence, and personalized care.
This clinical series reviews the data behind commonly used supplements and provides practical strategies for:
-
Evaluating supplement claims
-
Identifying when supplementation is truly beneficial
-
Selecting high-quality products
-
Avoiding unnecessary or potentially misleading interventions
By bridging the gap between supplement hype and scientific evidence, healthcare professionals can empower patients to make informed choices that support genuine health benefits rather than marketing promises.
News in the same category


Current Cardiac Screening Tools Miss Nearly Half of First Heart Attacks, Study Finds

Could Bone Broth Support Healthy Knee Cartilage as You Age?

Top 7 Best Drinks Diabetics Can Enjoy at Night to Support Healthy Blood Sugar Levels!

Papaya Leaves for Hair: A Natural Way to Support Healthier, Shinier Strands

Protect Your Eyes Naturally: 3 Powerful Seeds and 1 Fruit Every Senior Should Know About

Power Naps: The Benefits, How Long They Should Be, and When They Work Best
5 Early Signs and Symptoms of Ulcerative Colitis

8 Potential Health Benefits of Kombucha

Diagnosed with End-Stage Stomach Cancer, I Painfully Realized: 3 Foods Left Too Long in the Refrigerator Can Become “Accomplices” to Cancer

5 Detox Baths to Remove Aches, Pains and Toxins + Fragrant Bath Melts Recipe
Early Signs of Lung Disease & How to Strengthen Your Lungs

5 things you absolutely SHOULDN'T do in the morning if you don't want your cancer cells to "grow like wildfire"

Emerging Flu Variant ‘Subclade K’ Raises Global Health Concerns Across the US, UK, and Beyond

A New Alternative to Reading Glasses: Eye Drops for Age-Related Near-Vision Loss
Sip These 4 Crimson Nightcaps—Watch Creatinine Whisper Down While Your Kidneys Heal Overnight

Unlock Vibrant Aging with This Ruby-Red Hibiscus, Avocado & Clove Elixir

Heart Surgeon’s Hidden Secret: Eat This Daily to Boost Cardiac Health!

“Beer Belly” Fat May Damage the Heart Differently Than General Obesity, Study Suggests
News Post

Could a Simple Bedtime Drink with Three Common Ingredients Support Your Heart Health?

What kind of fruit is so familiar that most people eat it when it's ripe but don't know that the green fruit is considered the "architect" of the intestines?
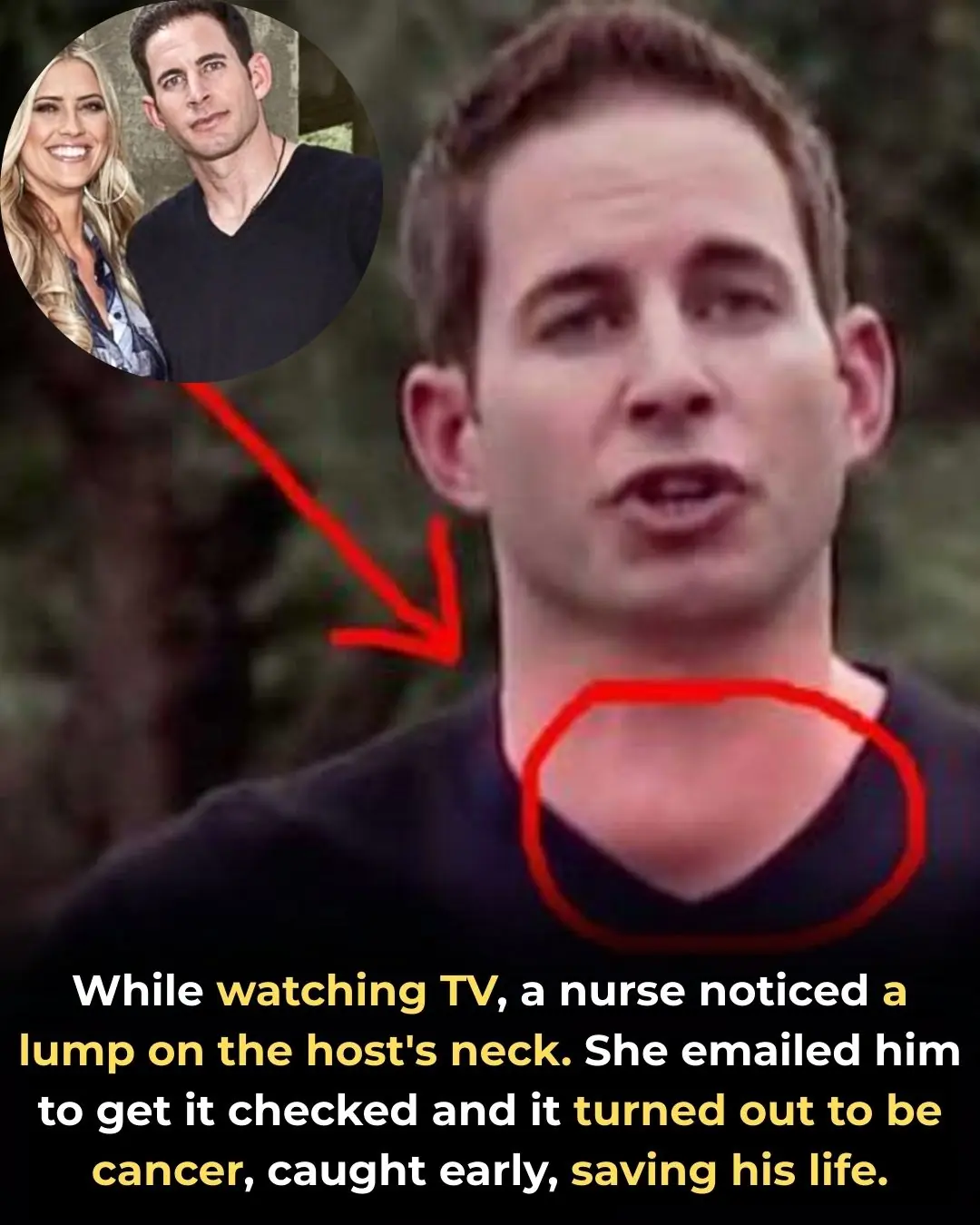
A TV Viewer’s Sharp Eye Helped Save Flip or Flop Star Tarek El Moussa’s Life

Graduate Kneels to Thank Brother Who Gave Up School for Her

32 Years of Service: Community Saves Elderly Carabao in the Philippines From Slaughter

A Father at Work, a Baby’s Smile, and a Moment That Touched Millions
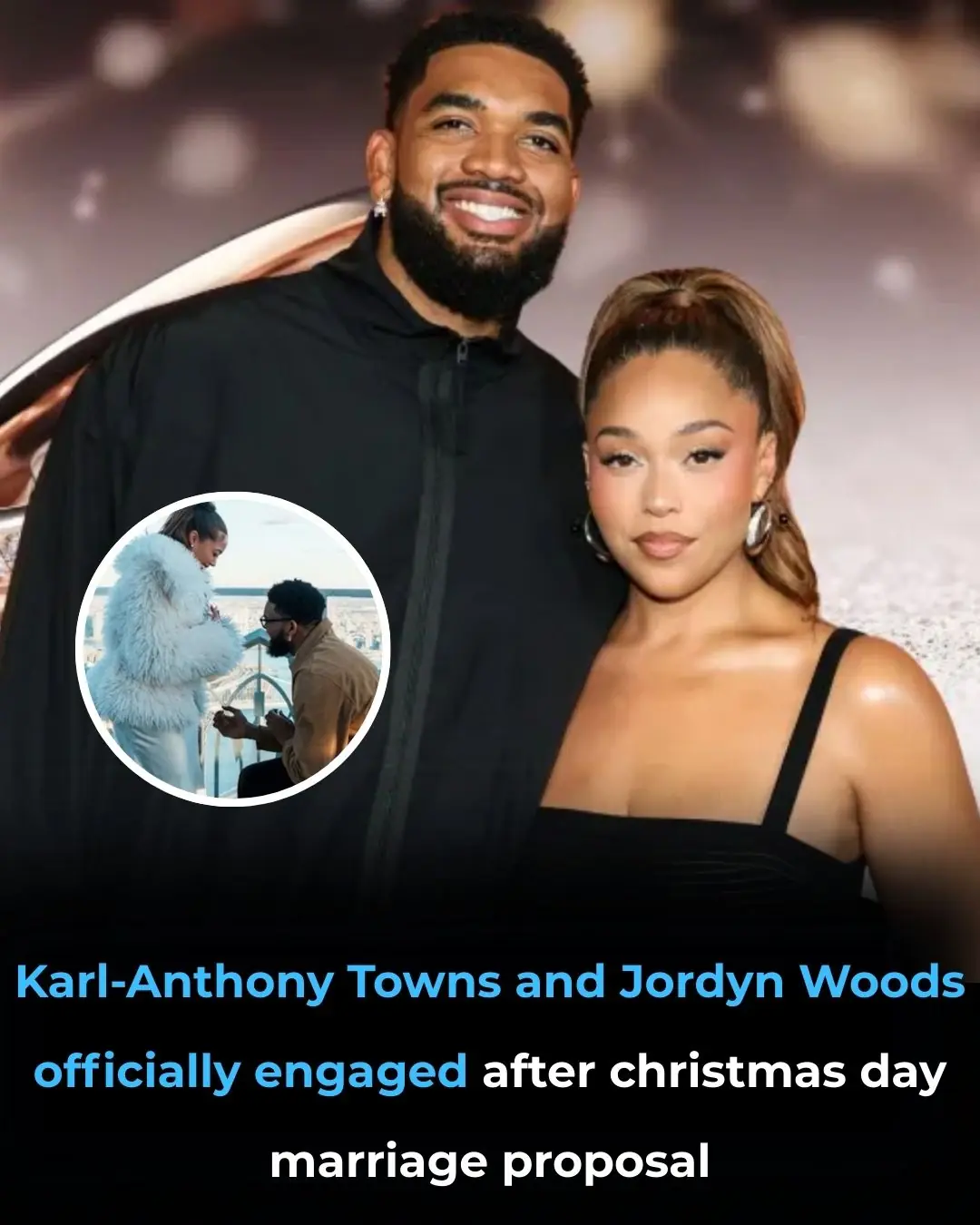
Jordyn Woods is engaged to Karl-Anthony Towns — see the massive engagement ring set to spark trends in 2026

A New Global Atlas Maps Every Building on the Planet in Unprecedented Detail

A Bed on Wheels: A Chinese Inventor’s Unusual Take on Personal Transportation

Planting Love That Endures: The Heart-Shaped Forest of Winston Howes

Current Cardiac Screening Tools Miss Nearly Half of First Heart Attacks, Study Finds

Canada Moves Toward Ending Captivity of Elephants and Great Apes

The Golden Retriever Who Became a Street View Star

A $5.7 Million Puppy: The Story of Cadabomb Okami

🐶 Why Dogs Can Watch TV

📚 What the New Curriculum Includes

Carols with Pets: A Festive Celebration of Faith, Music, and Companionship

Mosquitoes are most afraid of this bowl of water
