Promising Early-Phase Trial Suggests New KRAS-Targeted Vaccine Could Improve Outcomes in Pancreatic Cancer
Early-Phase Trial of a Novel Cancer Vaccine Shows Promise in Pancreatic Cancer Patients
A new therapeutic cancer vaccine, ELI-002 2P, has generated promising results in an early-phase clinical trial involving patients with pancreatic cancer, offering renewed hope in the fight against one of the most lethal malignancies. The vaccine is specifically designed to mobilize the body’s immune system against cancer cells harboring mutations in the KRAS gene, which are present in up to 90% of pancreatic cancers and are thought to drive tumor growth and progression.
The KRAS oncogene is notoriously difficult to target with conventional therapies. Changes in this gene lead to altered KRAS proteins that continuously signal cells to divide uncontrollably. By training the immune system to recognize these mutant proteins, ELI-002 2P aims to teach patients’ immune defenses to seek out and destroy cancer cells before they can establish new tumors or cause relapse.
In the phase 1 AMPLIFY-201 trial, patients who had undergone surgical removal of their pancreatic tumors received the vaccine as an adjuvant therapy—meaning it was given after surgery to help prevent the cancer from returning. The results showed that the vaccine was able to elicit strong T-cell immune responses, with approximately 84% of patients developing robust KRAS-specific T cells after vaccination. Those with stronger immune responses tended to have significantly longer periods without cancer recurrence and improved overall survival compared with those whose immune responses were weaker.
Importantly, the phase 1 study reported no dose-limiting toxicities or severe safety concerns, and the most common side effects were mild, such as fatigue and injection site reactions. These findings support the vaccine’s favorable safety profile at the doses tested.
Unlike many personalized cancer vaccines, which must be custom-made for each individual patient, ELI-002 2P is an “off-the-shelf” vaccine. This means it can be manufactured in advance, stored, and administered without the need for customization, potentially making it faster, more accessible and more cost-effective than bespoke vaccines tailored to individual tumor profiles.
The intermediate clinical data showed encouraging outcomes: median recurrence-free survival (RFS) in the treated cohort was around 16.3 months, and median overall survival (OS) was approximately 28.9 months, which compare favorably with historical outcomes in high-risk pancreatic cancer patients treated with standard therapy alone. Moreover, patients with particularly high immune responses to the vaccine showed even greater survival benefit, suggesting a strong correlation between immune activation and clinical outcomes.
While these results are preliminary, they are significant given the historically poor prognosis associated with pancreatic cancer. Many patients experience rapid relapse even after successful surgery and chemotherapy, and effective adjuvant therapies have been limited. The early data hint that a vaccine-based immunotherapy could reshape how pancreatic cancer is treated in the future.
Researchers caution, however, that larger phase 2 and phase 3 trials are necessary to confirm these findings and to fully establish the vaccine’s efficacy and long-term safety. Elicio Therapeutics, the biotechnology company developing ELI-002, is already advancing additional studies—including a phase 2 trial with a broader formulation targeting multiple KRAS mutations—which will provide more definitive evidence later this year.
If validated in larger populations, ELI-002 and similar KRAS-targeted immunotherapies could offer a powerful new tool against pancreatic cancer, a disease that has resisted many standard treatments and remains one of the leading causes of cancer death worldwide. Ongoing research suggests that combining such vaccines with other immunotherapies or conventional treatments may further improve patient outcomes in the future.
News in the same category

A New Cancer Vaccine Shows Long-Lasting Protection in Preclinical Studies

The Meaning of Having an Unmade Bed

Fatty liver disease: 6 symptoms you need to know

From NFL Stardom to Farming: Jason Brown’s Inspiring Journey to Fight Hunger and Honor His Brother
COVID-19 May Accelerate Blood Vessel Aging in Women, Study Finds

Scientists Unveil Hidden Ancient Landscape Beneath Antarctica’s Ice: A Glimpse into Earth’s Prehistoric Ecosystems
Study Finds Encouraging Immune Responses from Novel Pancreatic Cancer Vaccine

⚡ Power Unleashed: DARPA Transmits Energy Over 5 Miles Without Wires! 🔋✨

China Launches the Three-Body Computing Constellation: A New Era of AI-Powered Space-Based Supercomputing

Felix Baumgartner's Historic Jump: Breaking the Sound Barrier from the Edge of Space

Josie the Blind Lioness: A Remarkable Tale of Survival, Family Bonds, and Adaptation in Addo National Park

7 Red Flag Phrases Narcissists Use to Exert Control During Arguments

What Arriving Early All the Time Says About Your Personality, According to Psychology

If These 8 Activities Energize You Instead of Drain You, You’re Likely a Highly Intelligent Introvert

Sophia Park Makes History as the Youngest Person to Pass the California Bar Exam at 17

Legendary Japanese Actor Tatsuya Nakadai Passes Away at 92
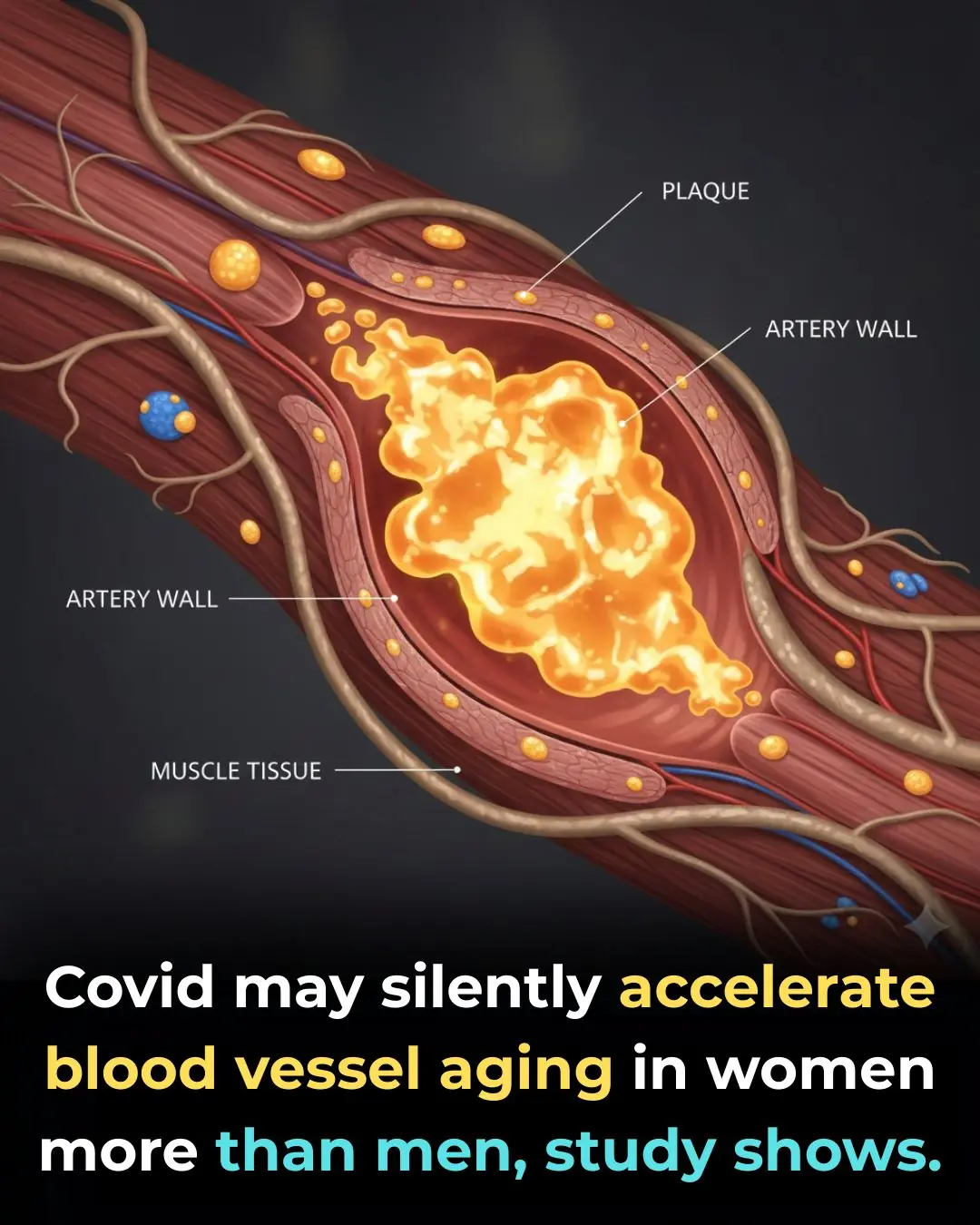
Even Mild Covid Can Age Women’s Arteries by Years, Scientists Warn

Too Much Screen Time in Toddlers May Damage Brain Growth and Delay Speech—Find Out Why

American Psychological Association (APA). Anxiety, control, and predictability.
News Post

Sleep Apnea: Symptoms, Risks, and Treatment Solutions

Always the Strong One: The Emotional Cost of Holding Everything Together

A New Cancer Vaccine Shows Long-Lasting Protection in Preclinical Studies
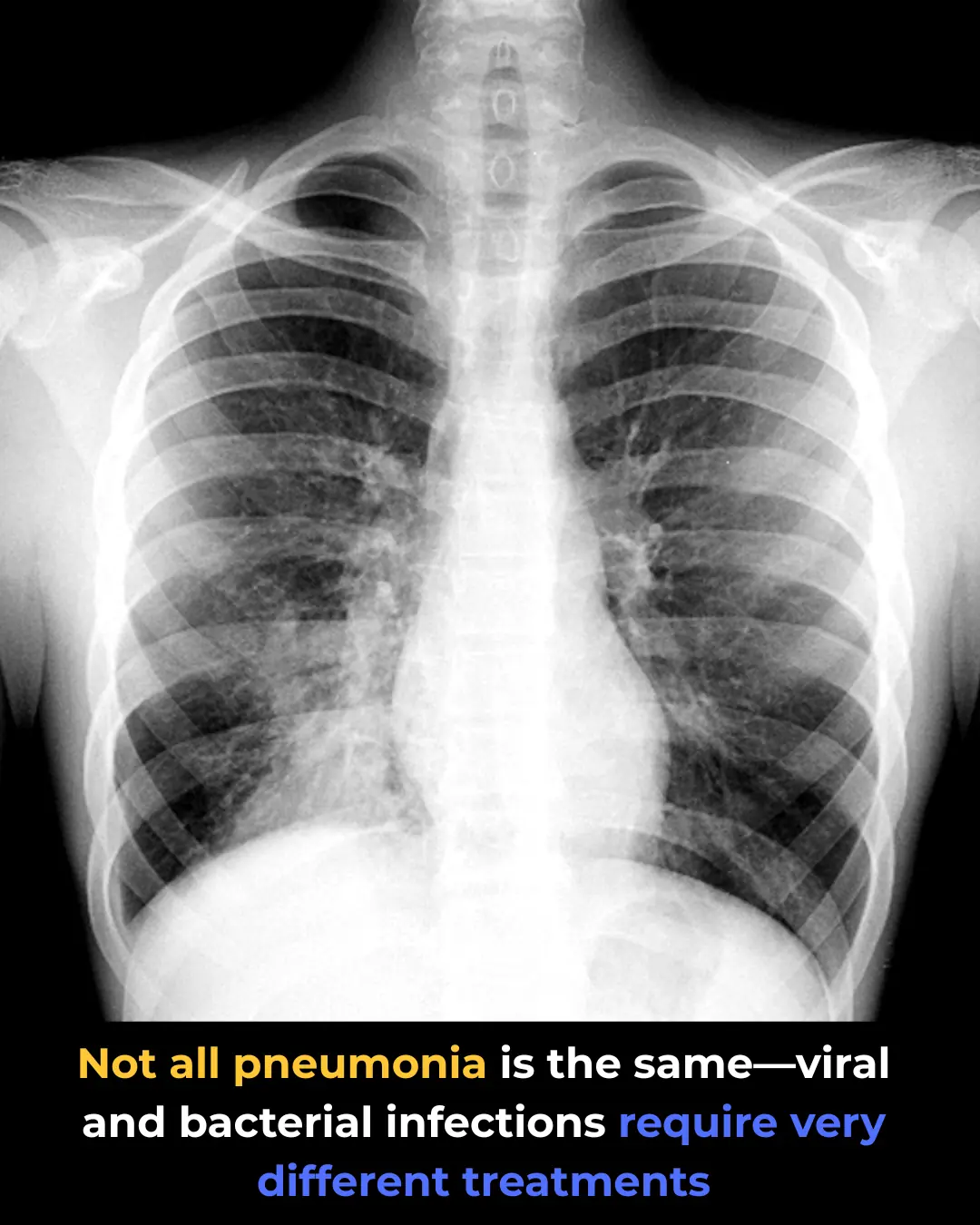
Viral Pneumonia vs. Bacterial Pneumonia: Key Differences

Allergic Rhinitis: What Triggers It and How to Manage It

COPD Exacerbation: Symptoms That Indicate a Flare-Up

Bile Reflux vs. Acid Reflux: Key Differences You Need to Know

Ulcerative Colitis vs. Crohn’s Disease: What Makes Them Different

How to lower blood sugar without giving up carbs

People Who Should Avoid Eating Kohlrabi (Su Hào), Even If They Really Crave It

Dip the rice noodles into a bowl of fish sauce; after 2 minutes, you'll know whether the noodles are clean or contain borax.

Dandelion: A “Superfood” Herb with Real Nutrients — What Science Says

Woman (26) Dies After Eating Hot Pot: 2 Things You Should Never Do Together When Enjoying Hot Pot

The grout lines of ceramic tiles are stained black; this method will clean them in just a few minutes, saving you the effort of scrubbing.

If the Body Is Developing Cancer, Three Nighttime Sleep Signs May Appear — But Many People Ignore Them

Desiccant packets have 8 special uses; throwing them away is like throwing money out the window.

Refrigerator door gaskets are covered in black mold? Use this to wipe them clean in just 5 minutes.

Don't throw away orange peels; this method has many uses that everyone needs, don't waste them.

Claim: “Cancer Cells Eliminated in 42 Days with a Special Juice — Worldwide Celebration?”